Common examples of oxidizing agents include halogens such as chlorine and fluorine oxygen and hydrogen peroxide H 2 O 2. An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction.

Answered A Choose The Item That Correctly Bartleby
The strongest oxidizing agent in the list is F2 followed by H2O2 and so on down to the weakest oxidizing agent Li.

. Cr changes from oxidation number 3 to 0. Oxidizing and Reducing Agents. A chemical reaction results in the rearrangement of the constituent atoms of the reactants to result in the formation of products.
Which best describes the oxidizing agent in this reaction. Asked By Wiki User. The Cr2O3 is the oxidizing agent.
An oxidizing agent often referred to as an oxidizer or an oxidant is a chemical species that tends to oxidize other substances ie. Oxidizing agents are also known as oxidants or oxidizers. Which best describes the oxidizing agent in this reaction.
It is a reactant that removes electrons from other reactants during a redox reaction. Which best identifies why the rusting of an iron nail in the presence of water and oxygen is an oxidation-reduction reaction. Reaction is as follow Cl ₂ aq 2 Br aq 2Cl aq Br ₂ aq Oxidation Reaction.
Which best describes the oxidizing agent in this reaction. The H2O2 is the oxidizing agent. Chlorine Cl is the oxidizing agent because it gains an electron Which of the following is true about a redox reaction.
D A substance that reacts with oxygen. Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron. O changes from oxidation number -1 to -2.
Heres a typical table of standard reduction potentials. Cause an increase in the oxidation state of the substance by making it lose electrons. It oxidizes the reducing agent.
The Cr2O3 is the oxidizing agent. Cl2aq 2Brmc003-1jpgaq mc003-2jpg 2Clmc003-3jpgaq Br2aq c. What is an Oxidizing Agent.
Which of the following is true about a redox reaction. An oxidizing agent is thus an electron acceptor. Which describes the oxidizing agent in a chemical reaction.
Chlorine Cl is the oxidizing agent because it gains an electron. The forming substances are called chemical elements or compounds. The oxidizing agent can have two meanings.
S changes from oxidation number -2 to 6. Hydrogen peroxide ozone oxygen potassium nitrate and nitric acid are all oxidizing agents. Which best describes an oxidizing agent.
It means a reactant which oxidizes other element or reactant ie it undergoes reduce themselves and gain an electron. The PbS is the reducing agent. Which of the following is an oxidation-reduction reaction.
The oxidizing agent typically takes these electrons for itself thus gaining electrons and being reduced. A process in which one or more reactants combine together to form one or more different substances called products. The oxidizing agent typically takes these electrons for itself thus gaining electrons and being reduced.
In a redox reaction an electron is lost by the reducing agent. Chlorine Cl is the oxidizing agent because it gains an electron. Chlorine Cl is the oxidizing agent because it gains an electron.
The CO is the reducing agent. In a redox reaction the oxidizing agent is reduced. C changes from oxidation number 4 to 2.
Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron. 2 Br Br₂ 2 e Two atoms of Br Bromide looses two electrons to form Br₂ molecule. Hence it is oxidized and is acting as reducing agent.

Solved A Choose The Item That Correctly Describes The Chegg Com

Answered A Choose The Item That Correctly Bartleby
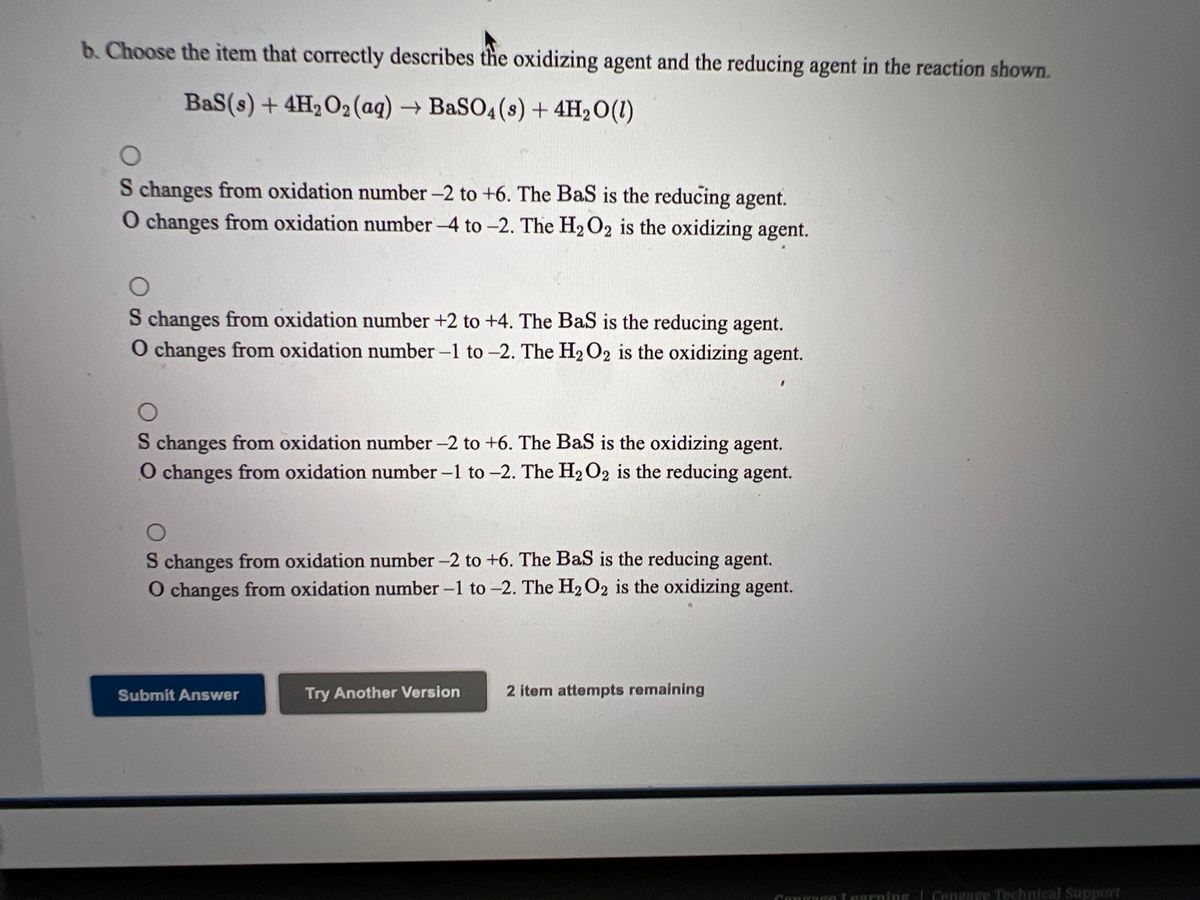
Answered A Choose The Item That Correctly Bartleby

Solved The Reaction Of Copper And Silver Nitrate Is Chegg Com
0 Comments